Overview
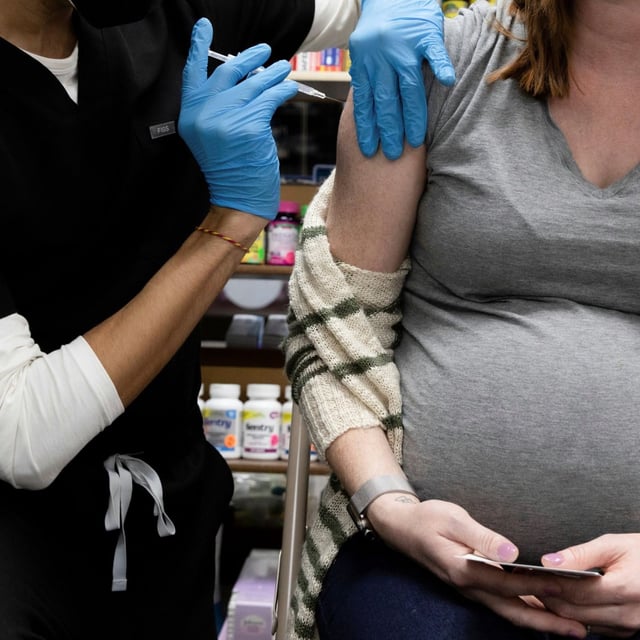
- HHS Secretary Robert F. Kennedy Jr. announced that the CDC will no longer recommend COVID-19 vaccines for healthy children and pregnant women.
- Last week’s FDA framework confines routine approvals to adults aged 65 and older or individuals with underlying conditions and requires placebo-controlled trials for healthy under-65s.
- The abrupt policy shift bypassed the CDC’s Advisory Committee on Immunization Practices, which is still scheduled to meet in June to set guidelines for fall boosters.
- Removal from the CDC schedule means private insurers and government programs like Medicaid will no longer be required to cover vaccines for these groups.
- Health experts warn that pregnant women face higher risk of severe COVID-19 and that pausing routine childhood vaccinations may affect pediatric hospitalization rates.