Overview
- The FDA has cleared Fujirebio Diagnostics' Lumipulse blood test, the first approved blood-based diagnostic for Alzheimer’s disease.
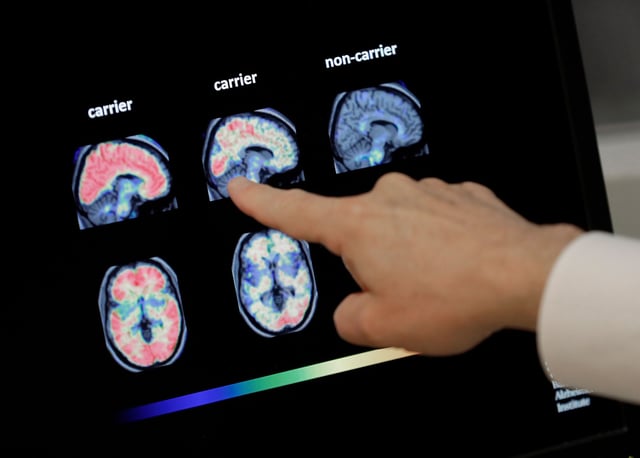
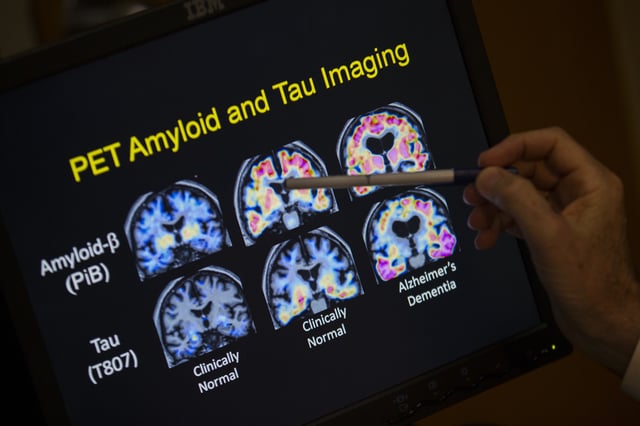
- The test measures the ratio of two proteins, pTau217 and β-amyloid 1-42, to detect amyloid plaques associated with Alzheimer’s.
- Authorized for patients aged 55 and older with cognitive decline, the test demonstrated over 97% accuracy for negative results and 91.7% for positive results in clinical studies.
- Lumipulse provides a less invasive alternative to PET scans and spinal taps, potentially streamlining early diagnosis and access to amyloid-targeting therapies like Leqembi and Kisunla.
- The test is expected to initially serve as a tool to rule out Alzheimer’s, with positive results requiring confirmatory testing before a final diagnosis.