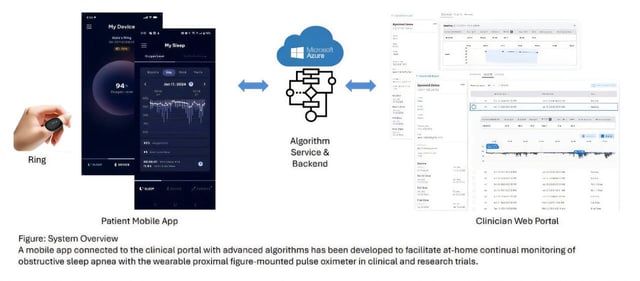
Overview
- Clinical trials presented at ATS 2025 highlight robust patient adherence, with 85% exceeding recommended usage and one patient wearing the device for 44 days.
- The device uses advanced transmittance-based photoplethysmography for accurate oxygenation monitoring, even in low perfusion states.
- Researchers suggest multi-night monitoring with fewer data channels could rival traditional in-lab polysomnography for assessing OSA treatment response.
- The wearable connects to a smartphone app, allowing patients and providers to share data and collaborate on treatment plans.
- While the pulse oximeter is FDA-cleared, the connected software platform requires regulatory approval before broader clinical deployment.