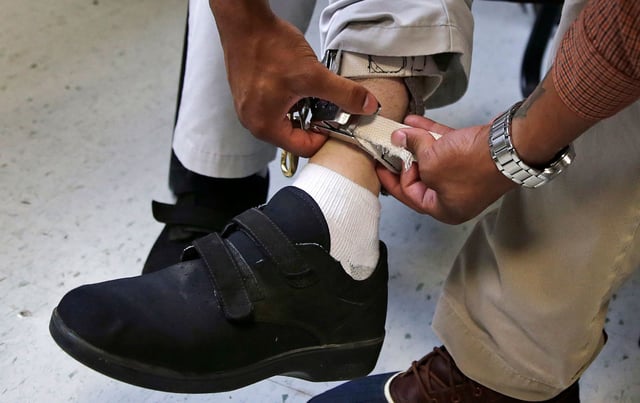
Overview
- The FDA aims to ban electric shock devices at the Judge Rotenberg Education Center, the only school in the U.S. using them for behavior management.
- This marks the second attempt by the FDA to prohibit these devices, following a federal court's overturn of a similar ban in 2020.
- The devices, intended to curb aggressive or self-harming behavior, have been criticized for posing psychological and physical risks.
- Legal and regulatory changes since the initial ban now clarify the FDA's authority to enforce such prohibitions.
- The Judge Rotenberg Education Center defends the treatment as 'life-saving,' despite widespread condemnation and legal challenges.