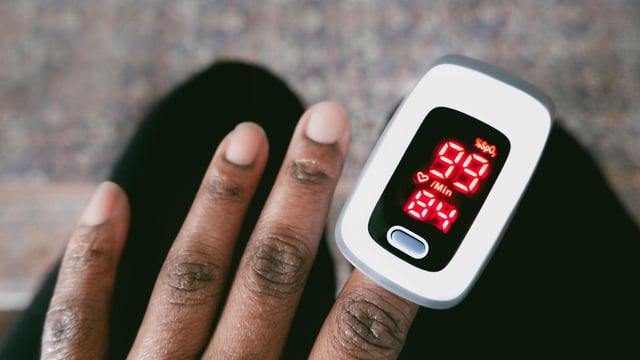
Overview
- The FDA advisory panel met to discuss making pulse oximeters more accurate for people with darker skin, following research indicating significant inaccuracies.
- Research has shown that pulse oximeters often provide incorrect readings for darker skin tones, leading to treatment delays and worse health outcomes for minorities.
- Over 20 state attorneys general have urged the FDA to update guidelines to include warnings about pulse oximetry’s inaccuracy on darker skin.
- The FDA issued a warning in 2021 about the limitations of pulse oximeters, highlighting their risk of inaccuracy under certain conditions.
- Experts emphasize the need for devices that do not discriminate based on skin color, advocating for health equity and the development of more inclusive medical devices.