Overview
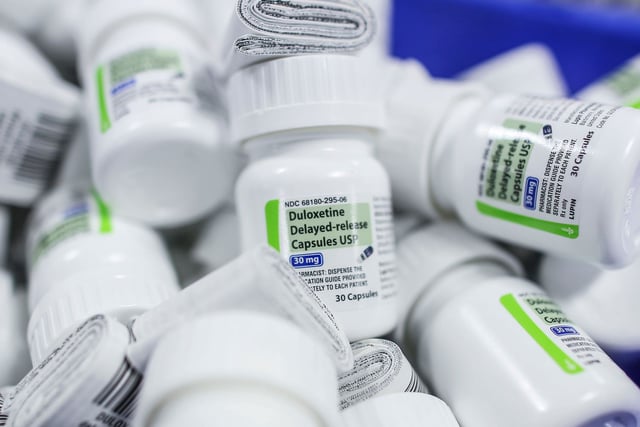
- The recall affects duloxetine, commonly known as Cymbalta, used to treat depression and anxiety.
- The presence of N-nitroso-duloxetine, a nitrosamine impurity, was found above acceptable limits, prompting the recall.
- The recall is classified as Class II, indicating the potential for temporary or medically reversible adverse health effects.
- Towa Pharmaceutical Europe, the manufacturer, is collaborating with the FDA and other regulators to manage the recall.
- Patients are advised to consult healthcare professionals before discontinuing use of the affected medication.