Overview
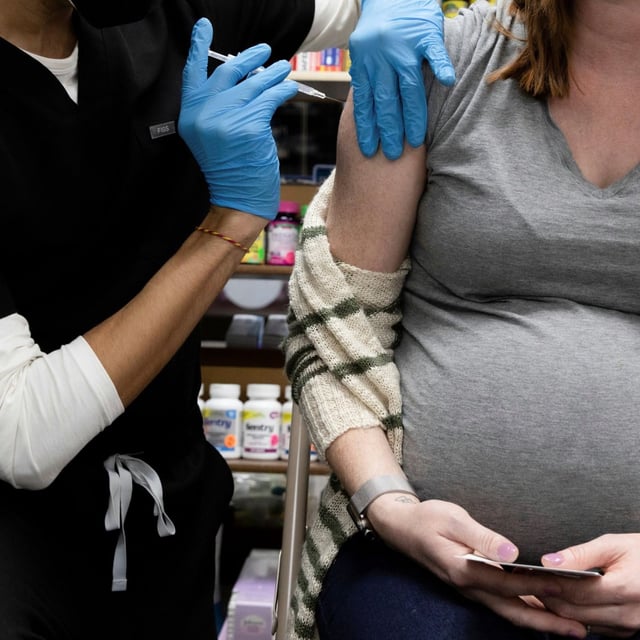
- On May 27, Health Secretary Robert F. Kennedy Jr. announced that healthy children and pregnant women will be dropped from the CDC’s recommended COVID-19 immunization schedule.
- Kennedy’s decision sidestepped the CDC’s Advisory Committee on Immunization Practices, which is scheduled to evaluate fall vaccine guidance in June.
- Last week the FDA narrowed routine COVID-19 vaccine approvals to seniors and people with underlying conditions, requiring new clinical trials for other groups.
- Health experts warn insurers and programs like Medicaid may no longer be obligated to cover shots for children and pregnant women, reducing access.
- Infectious disease specialists emphasize that pregnant women face higher risks of severe COVID-19 and that vaccination benefits newborns and young children.