Overview
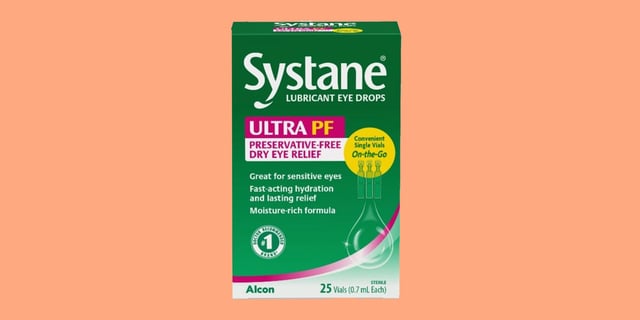
- The recall affects Lot 10101 of Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, with an expiration date of September 2025.
- The contamination was discovered after a consumer reported foreign material in a sealed vial, later identified as fungal in nature.
- The FDA warns that fungal contamination in eye products can cause serious eye infections, potentially threatening vision or life in rare cases for immunocompromised individuals.
- No adverse reactions or infections have been reported to date, and the recall is being conducted as a precautionary measure.
- Consumers are advised to stop using the recalled product immediately and return it to the place of purchase for a refund or replacement.