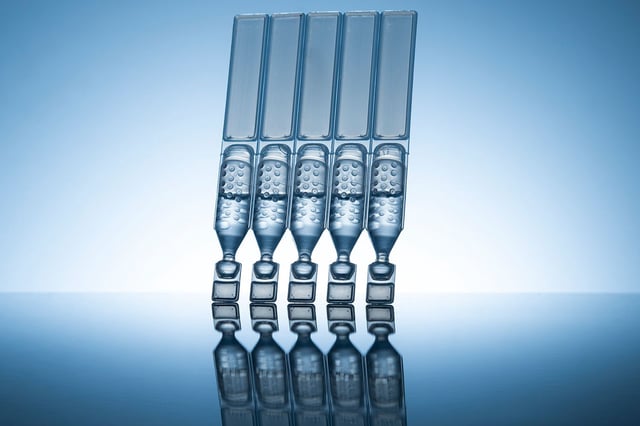
Overview
- The recall affects Systane Lubricant Eye Drops Ultra PF, sold in 25-count single-use vials with lot number 10101 and an expiration date of September 2025.
- The contamination, determined to be fungal in nature, was reported by a customer who observed foreign material in a sealed vial.
- Fungal contamination in eye drops can cause serious eye infections, potentially leading to vision loss or life-threatening conditions in immunocompromised individuals.
- No adverse events or infections have been reported so far, but Alcon has initiated the recall out of caution and is investigating the issue.
- Consumers are advised to stop using the affected product immediately and return it to the place of purchase for a refund or replacement.